Bone tissue is a highly dynamic organ that relies on constant communication among diverse cell types to maintain structural integrity and metabolic homeostasis. This article delves into the complex networks of signaling pathways and intercellular interactions that regulate bone formation, resorption, and adaptation to mechanical stress. By examining molecular cues and communication routes, researchers aim to uncover novel therapeutic targets for osteoporosis, fracture healing, and other skeletal disorders.
Bone Tissue Dynamics: Cellular Players and Structural Framework
Bone remodeling involves a balanced cycle of resorption and formation driven by specialized cells within the bone matrix. Osteoclasts are large, multinucleated cells responsible for breaking down mineralized bone. They adhere to the bone surface, creating resorption pits through the secretion of acid and proteolytic enzymes. Conversely, osteoblasts synthesize new bone matrix, secreting collagen type I and orchestrating mineral deposition. A third population, osteocytes, are former osteoblasts embedded within lacunae. These cells extend dendritic processes through canaliculi, forming an extensive lacuno-canalicular network that senses mechanical load and regulates both osteoclast and osteoblast activity.
The extracellular matrix serves as a scaffold and signaling reservoir. It contains embedded growth factors such as bone morphogenetic proteins (BMPs) and insulin-like growth factors (IGFs), which influence cellular proliferation and differentiation. Non-collagenous proteins like osteopontin and osteocalcin also modulate mineral nucleation and cell adhesion. The interplay between matrix composition and cellular receptors ensures that bone tissue can adapt to physiological demands, respond to injury, and maintain systemic calcium levels.
Signaling Pathways Governing Bone Homeostasis
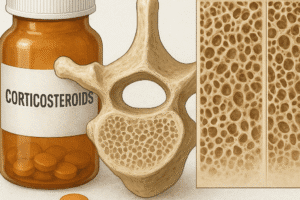
A finely tuned network of molecular signals regulates bone remodeling. One of the most studied axes is the RANK/RANKL/OPG pathway. RANKL (Receptor Activator of Nuclear Factor κB Ligand) is expressed by osteoblast lineage cells and stromal cells. It binds to its receptor RANK on osteoclast precursors, promoting differentiation and activation. Osteoprotegerin (OPG) acts as a decoy receptor, sequestering RANKL and preventing excessive bone resorption. Dysregulation of this axis leads to osteoporosis or osteopetrosis, depending on the balance between RANKL and OPG.
Another critical cascade is the Wnt/β-catenin pathway. When Wnt proteins bind to Frizzled receptors and co-receptors LRP5/6 on osteoblasts, β-catenin accumulates in the cytoplasm and translocates to the nucleus, stimulating genes that drive osteoblast proliferation and matrix production. Antagonists like sclerostin and DKK1 inhibit Wnt signaling, reducing bone formation. Monoclonal antibodies targeting sclerostin have demonstrated anabolic effects in clinical trials, highlighting the translational potential of manipulating Wnt signals.
Notch signaling also influences bone cell fate. Interaction between Notch receptors and ligands on adjacent cells triggers proteolytic cleavage of the Notch intracellular domain, which translocates to the nucleus to regulate gene expression. Notch activity in osteoblasts can either promote proliferation or, in later stages, inhibit differentiation, illustrating the context-dependent nature of this pathway.
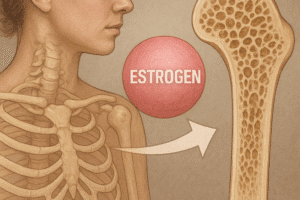
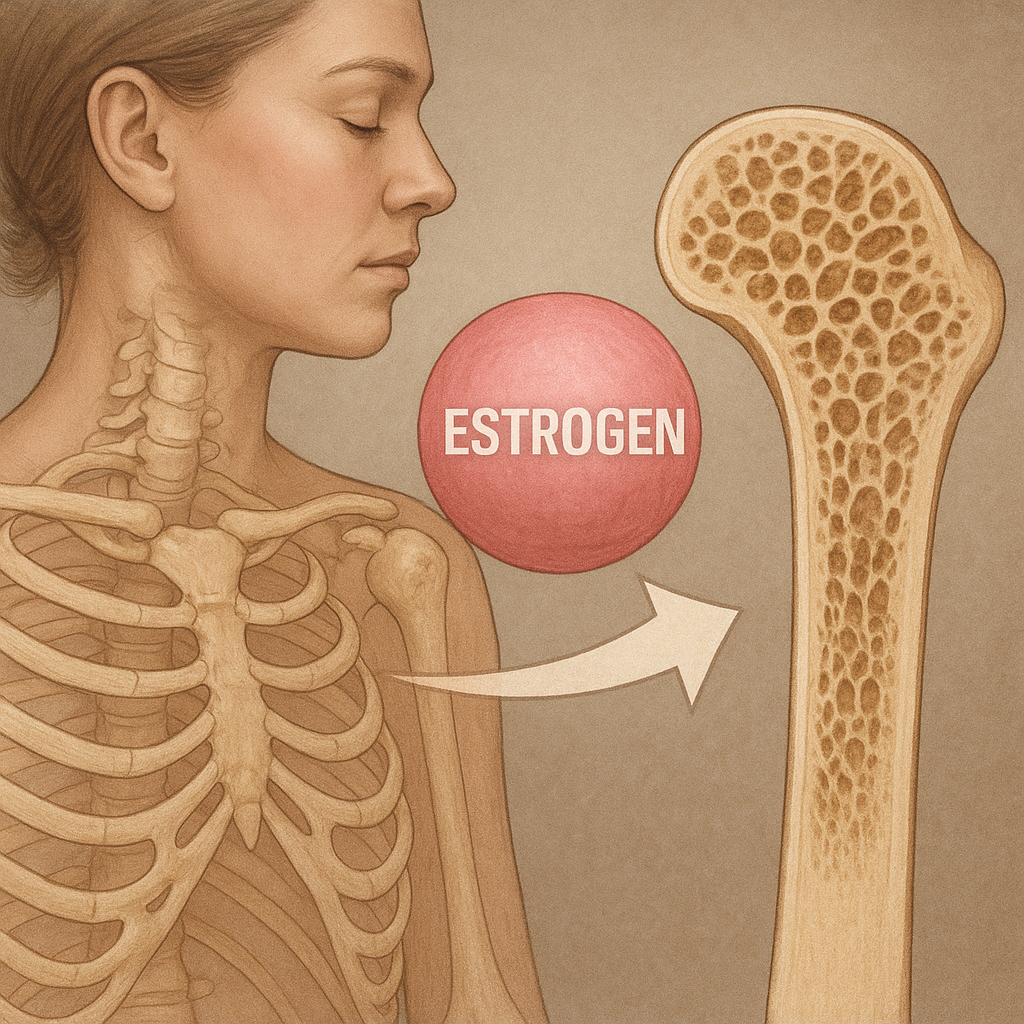
Gap junctions composed of Connexin 43 facilitate direct cytoplasmic exchange of ions and metabolites between osteocytes and other bone cells. Through these junctions, cells coordinate responses to hormonal stimuli such as parathyroid hormone (PTH) and estrogen. PTH intermittently enhances osteoblastic bone formation, whereas chronic elevation can lead to bone loss. Estrogen deficiency, as seen in menopause, increases RANKL expression and osteoclast activity, underscoring hormonal regulation in skeletal health.
Mechanotransduction and Extracellular Vesicle Communication in Bone
Bone adapts to mechanical forces through mechanotransduction, the conversion of physical stimuli into biochemical signals. Osteocytes play a pivotal role by sensing shear stress in the lacuno-canalicular system. Fluid flow stimulates ion channels, integrins, and primary cilia, triggering downstream cascades including MAPK and PI3K/Akt signaling. These events regulate the expression of genes that control bone formation and resorption, aligning bone architecture with mechanical demands.
Recent discoveries highlight the importance of extracellular vesicles (EVs) in bone communication. Osteoblasts, osteocytes, and osteoclasts release exosomes and microvesicles loaded with microRNAs, proteins, and lipids. For example, osteoclast-derived vesicles enriched in miR-214 can inhibit osteoblast activity, while osteoblast-derived exosomes carrying osteogenic miRNAs promote osteogenesis in recipient cells. EV-mediated crosstalk provides both local and systemic avenues for regulating bone remodeling and may serve as biomarkers or therapeutic vectors.
Inflammatory cytokines such as TNF-α and IL-6, produced by immune cells or the bone cells themselves, further modulate remodeling under pathological conditions. Chronic inflammation can tilt the balance toward resorption, contributing to bone loss in rheumatoid arthritis and other metabolic diseases. Targeting these cytokines has shown promise in preserving bone mass, revealing another layer of intercellular communication that intersects with classic signaling pathways.
By integrating mechanical cues, hormone levels, cytokine networks, and vesicle-mediated exchanges, the bone microenvironment maintains a delicate equilibrium. Future research into these communication routes holds the potential to develop innovative treatments for skeletal disorders, enhance fracture repair, and improve outcomes for patients affected by bone-related diseases.