Bone health is a dynamic interplay of cellular activities, genetic predispositions, and endocrine influences. Estrogen, a key ovarian hormone, exerts potent effects on skeletal homeostasis, modulating bone remodeling, mineralization, and structural integrity. This article explores multiple dimensions of estrogen’s impact on the adult skeleton, from molecular signaling pathways to clinical approaches aimed at preserving bone density and preventing debilitating conditions such as osteoporosis.
Physiological Effects of Estrogen on Bone Metabolism
During the reproductive years, adequate levels of estrogen maintain a fine balance between bone formation and resorption. This balance is orchestrated by two primary cell types:
- Osteoblasts, responsible for new bone synthesis and mineral deposition.
- Osteoclasts, which degrade old or damaged bone tissue.
Under normal conditions, estrogen inhibits the recruitment and activity of osteoclasts, thereby reducing bone resorption. Concurrently, it promotes osteoblast differentiation and prolongs their lifespan, enhancing bone formation. These combined actions support net bone accrual and the maintenance of microarchitectural integrity.
Regulation of Bone Remodeling Cycle
Bone remodeling is a continuous process involving resorption, reversal, and formation phases. Estrogen influences each phase:
- Resorption: By decreasing expression of RANKL (Receptor Activator for Nuclear Factor κ B Ligand) in osteoblasts, estrogen limits osteoclastogenesis.
- Reversal: It modulates macrophage-like cells to create an environment conducive to osteoblast recruitment.
- Formation: Through upregulation of growth factors such as IGF-1 and TGF-β, estrogen enhances osteoblast function and matrix synthesis.
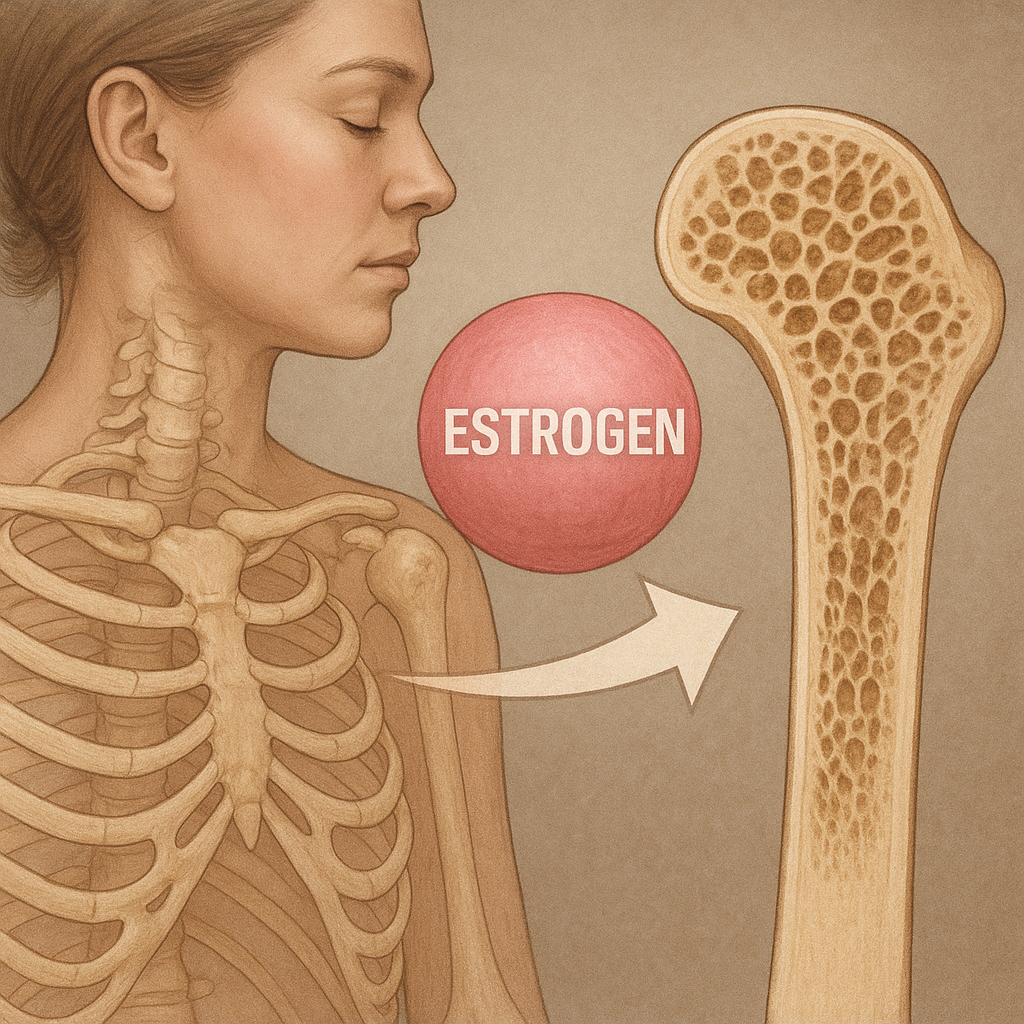
Experimental models demonstrate that estrogen deficiency accelerates bone turnover, leading to cortical thinning and trabecular perforation. This is particularly evident in postmenopausal women, where the abrupt decline in estrogen triggers a rapid phase of bone loss.
Mechanisms of Action at Cellular Level
At the molecular level, estrogen signals through two major receptor isoforms: ERα and ERβ. These nuclear receptors modulate gene transcription and interact with membrane-associated pathways, providing both genomic and nongenomic effects on bone cells.
Genomic Pathways
In the classical genomic mechanism, estrogen binds to ERα or ERβ in the cytoplasm. The hormone-receptor complex dimerizes and translocates to the nucleus, where it binds to estrogen response elements (EREs) on target genes. Key bone-related genes regulated by this mechanism include:
- OPG (Osteoprotegerin), which acts as a decoy receptor for RANKL and inhibits osteoclast development.
- ALP (Alkaline Phosphatase), a marker of osteoblast maturation and matrix mineralization.
- Sclerostin, produced by osteocytes to inhibit bone formation; estrogen suppresses its expression.
Nongenomic Pathways
Rapid estrogen effects involve membrane-bound receptors and activation of kinase cascades such as PI3K/Akt and MAPK. These pathways influence cytoskeletal dynamics, calcium signaling, and apoptosis resistance in bone cells:
- Enhanced osteoblast survival through Akt-mediated inhibition of pro-apoptotic factors.
- Modulation of NF-κB activity to reduce expression of pro-resorptive cytokines and inflammation mediators.
- Cross-talk with Wnt/β-catenin signaling to synergistically promote bone formation.
Clinical Implications and Therapeutic Strategies
Understanding estrogen’s multifaceted role in bone biology underpins various clinical interventions aimed at preventing or treating osteoporosis. These strategies can be pharmacological, lifestyle-based, or surgical.
Hormone Replacement Therapy (HRT)
HRT remains a cornerstone for managing postmenopausal bone loss. By restoring circulating estrogen levels, HRT:
- Reduces bone turnover and increases bone density by up to 10% within several years.
- Lowers fracture risk in the spine and hip by 30–50%.
- Mitigates menopausal symptoms such as hot flashes, enhancing patient adherence to therapy.
However, HRT use must be individualized, considering risks such as thromboembolism and breast cancer. Low-dose and transdermal formulations offer favorable safety profiles for long-term use in eligible candidates.
Selective Estrogen Receptor Modulators (SERMs)
SERMs, such as raloxifene and bazedoxifene, mimic estrogen’s beneficial skeletal effects while antagonizing its action in breast and uterine tissues. Advantages include:
- Prevention of vertebral fractures without stimulating endometrial proliferation.
- Favorable lipid profile modulation, reducing LDL cholesterol levels.
- No significant increase in breast cancer incidence compared to placebo.
Nonetheless, SERMs may not be as potent as HRT in preserving total hip density and can be associated with hot flashes and leg cramps.
Nonhormonal Alternatives
For patients with contraindications to estrogen-based therapies, other agents are available:
- Bisphosphonates: Inhibit osteoclast-mediated resorption and are effective in reducing vertebral and nonvertebral fractures.
- Denosumab: A monoclonal antibody against RANKL, offering reversible suppression of bone resorption with a convenient dosing schedule.
- Teriparatide: A recombinant PTH analog that stimulates bone formation when administered intermittently.
These options can be combined or sequenced based on fracture risk, renal function, and patient preferences, providing personalized approaches to osteoporosis management.
Influence of Menopause and Future Directions
Menopause marks a critical window for bone health, as estrogen levels plummet and bone loss accelerates. Early identification of at-risk women through bone density screening and assessment of clinical risk factors is essential. Emerging areas of research include:
- Development of tissue-selective estrogen complexes to maximize skeletal benefits while minimizing adverse effects.
- Gene therapy targeting estrogen receptor coactivators to enhance bone-specific transcriptional activity.
- MicroRNA-based interventions designed to modulate osteoblast-osteoclast coupling and inflammatory pathways in the bone microenvironment.
Advances in imaging and biomarker analytics will also refine patient stratification, enabling timely initiation of preventive measures.
Lifestyle and Nutritional Considerations
Adjunctive measures such as adequate calcium and vitamin D intake, weight-bearing exercise, and smoking cessation further support estrogen’s protective effects. Regular physical activity not only stimulates mechanotransduction pathways in bone but also improves muscle strength and balance, reducing fall risk.
Figure Legends (if applicable): Diagrams illustrating estrogen signaling pathways in osteoblasts and osteoclasts; comparative graphs of bone density changes with various therapies.
Key Terms: estrogen, osteoblast, osteoclast, bone density, menopause, hormones, receptor, inflammation, signaling, osteoporosis