The Future of Personalized Bone Medicine explores a transformative horizon where diagnostics, treatments, and patient management converge through cutting-edge science. By harnessing the power of genomics, advanced imaging, and AI-driven analytics, clinicians aim to deliver tailor-made bone health solutions. Innovations in biotechnology, biomaterials, and digital health platforms are unlocking unprecedented avenues for prevention, repair, and lifelong skeletal maintenance.
Technological Innovations Driving Personalized Bone Care
Rapid progress in imaging modalities and data processing is reshaping how bone disorders are detected and monitored. Traditional methods like dual-energy X-ray absorptiometry (DEXA) still play a critical role, but emerging techniques offer far deeper insight into bone quality and microarchitecture.
- High-resolution peripheral quantitative computed tomography (HR-pQCT) provides three-dimensional analysis of trabecular and cortical compartments, enabling early identification of fracture risk beyond bone mineral density alone.
- Ultrasound elastography captures mechanical properties of bone and surrounding soft tissue, offering a radiation-free alternative for frequent monitoring.
- Integration of sensor-equipped devices such as wearable accelerometers supports long-term activity tracking, complementing clinical assessments with real-world movement data.
Central to these advancements is the growth of digital health ecosystems. Cloud-based platforms collect and analyze patient data, fueling machine-learning algorithms that predict individual outcomes and suggest optimized intervention timelines. The integration of patient-generated data with electronic health records transforms episodic consultations into continuous, adaptive care pathways.
Integrative Approaches in Bone Regeneration and Repair
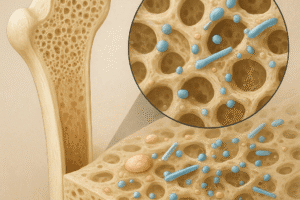
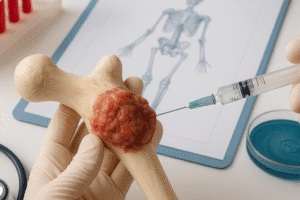
Personalized bone repair hinges on the triad of stem cell technology, biomaterials, and localized delivery systems. Recent breakthroughs are aligning these elements into cohesive regenerative therapies aimed at restoring bone integrity after trauma, tumor resection, or degenerative disease.
Stem Cell and Growth Factor Synergies
- Mesenchymal stem cells derived from adipose tissue or bone marrow, when guided by targeted growth factors, demonstrate enhanced osteogenic potential for critical-size defects.
- Scaffold-free spheroids enriched with bone morphogenetic proteins exploit cellular self-organization to generate vascularized bone constructs in vitro.
Advanced Biomaterials and 3D Printing
- Customizable implants manufactured via 3D printing enable patient-specific geometries that fit defect sites precisely, reducing surgical time and improving stability.
- Biodegradable composites combining ceramics, polymers, and nanotechnology elements release therapeutic payloads over controlled intervals, promoting stepwise tissue regeneration.
Efforts to engineer “smart” scaffolds with embedded sensors are underway, allowing real-time monitoring of pH, oxygen levels, and mechanical load at the repair site. These sensor-laden matrices feed data back to clinicians, who can adjust pharmacotherapy, physical therapy protocols, or scaffold design for optimal outcomes.
Data-Driven Medicine: From Genomics to AI
The integration of personalized molecular profiles with predictive analytics marks the apex of modern bone medicine. By combining genomic, proteomic, and metabolomic datasets, researchers identify novel biomarkers that flag early-stage osteoporosis or atypical fracture patterns.
- Population-scale genome-wide association studies (GWAS) continuously refine risk alleles linked to low bone density, informing screening recommendations for high-risk groups.
- Single-cell sequencing elucidates cell heterogeneity within bone marrow niches, revealing targets for enhanced osteoblast and osteoclast modulation.
- Computational bioinformatics pipelines process multi-omic inputs to stratify patients into molecular subtypes with distinct therapeutic sensitivities.
Artificial intelligence tools scrutinize imaging scans, electronic health records, and wearable data streams to produce dynamic risk models. Such AI-driven analytics can forecast fracture risk months in advance, allowing preemptive lifestyle modifications or pharmacologic interventions. Moreover, digital twins—virtual replicas of patient physiology—simulate treatment responses, enabling clinicians to test scenarios in silico before committing to invasive procedures.
Ethical and Regulatory Considerations
As bone medicine becomes more intricate, governance frameworks must evolve to safeguard patient rights and ensure equitable access to innovative care. Key issues include data privacy, informed consent for genomic testing, and the validation of AI algorithms.
- Data security: Protecting sensitive genetic and activity data against breaches is paramount to maintain patient trust and comply with regulations such as GDPR and HIPAA.
- Transparency: Patients must receive clear explanations of how algorithms weigh variables, especially when screening outcomes influence insurance coverage or surgical eligibility.
- Regulatory harmonization: Global alignment on standards for regenerative products, digital devices, and companion diagnostics is needed to accelerate clinical approval while preserving safety.
Equity in access remains a major challenge. High costs associated with personalized therapies and advanced diagnostics may widen disparities in bone health outcomes. Stakeholders—including policymakers, payers, and patient advocacy groups—must collaborate to implement pricing models, reimbursement strategies, and public-private partnerships that democratize these breakthroughs.