Bone tissue regeneration represents a transformative frontier in orthopedics and tissue engineering, driven by innovations in growth factors and biomaterials. The integration of molecular signals, scaffold design, and cellular therapies aims to restore bone integrity after trauma, disease, or congenital defects. This article examines the fundamental biology of bone repair, explores cutting-edge delivery platforms for key proteins, and highlights clinical successes alongside emerging challenges.
Mechanisms of Bone Healing and Role of Growth Factors
Bone repair unfolds through a well-orchestrated sequence of biological events. Understanding the cellular and molecular phases lays the foundation for targeted interventions. Central to this process are mesenchymal stem cells (MSCs) and a milieu of signaling proteins that together drive osteogenesis and angiogenesis.
Phases of Bone Repair
- Inflammatory Phase: Within hours of injury, hematoma formation recruits immune cells that release cytokines, cleaning debris and priming the microenvironment.
- Reparative Phase: MSCs differentiate into chondrocytes and osteoblasts. A soft callus forms, later mineralizing into woven bone.
- Remodeling Phase: Woven bone is gradually replaced by lamellar bone, restoring mechanical strength and microarchitecture.
Key Growth Factors in Osteogenesis
Several endochondral signaling proteins orchestrate stem cell recruitment, proliferation, and matrix deposition. Among the most studied are:
- Bone Morphogenetic Proteins (BMPs): Particularly BMP-2 and BMP-7, which induce MSC differentiation toward osteoblastic lineage.
- Vascular Endothelial Growth Factor (VEGF): Promotes formation of new blood vessels, ensuring nutrient supply and waste removal.
- Transforming Growth Factor-Beta (TGF-β): Regulates extracellular matrix synthesis and modulates inflammation.
- Platelet-Derived Growth Factor (PDGF): Enhances cell migration and proliferation, facilitating early callus formation.
Strategies for Controlled Delivery of Growth Factors
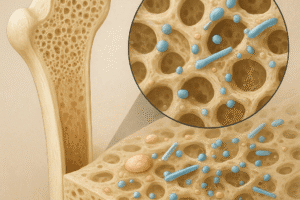
Effective translation of these proteins into clinical practice hinges on controlled release systems that maintain bioactivity and spatiotemporal presentation. A successful delivery platform must balance biocompatibility, mechanical support, and appropriate degradation rates.
Synthetic vs. Natural Scaffolds
- Polymeric Scaffolds: Materials like poly(lactic-co-glycolic acid) (PLGA) offer tunable degradation but may require surface modification to enhance cell adhesion.
- Hydrogels: Composed of natural polymers (collagen, hyaluronic acid) or synthetic derivatives, hydrogels can encapsulate growth factors for diffusive release.
- Ceramic-based Matrices: Calcium phosphate and bioactive glass mimic bone mineral content, providing innate osteoconduction.
- Composite Constructs: Combining polymers with ceramics yields scaffolds that merge flexibility with structural integrity.
Release Kinetics and Carrier Design
Release profiles can be tailored using various techniques:
- Physical Encapsulation: Entrapping growth factors within polymer matrices enables an initial burst followed by sustained diffusion.
- Covalent Binding: Tethering proteins to scaffold surfaces prolongs retention but requires careful chemistry to preserve activity.
- Nanoparticles and Microparticles: Biodegradable particles loaded with BMPs or VEGF can be embedded in a larger construct, achieving multi-stage delivery.
- Layer-by-Layer Assembly: Alternate deposition of polyelectrolytes and proteins produces stratified release patterns.
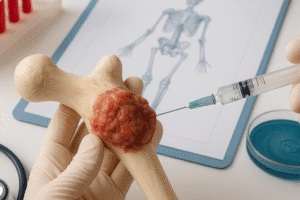
Clinical Applications and Future Directions
Integrating growth factor–based therapies into orthopedic and craniofacial surgery has yielded promising outcomes, yet several hurdles remain. Key considerations include dose optimization, cost-effectiveness, and regulatory pathways.
Orthopedic and Spinal Fusion Procedures
BMP-2 has been approved for select applications, such as lumbar spinal fusion and tibial nonunion. Clinical trials demonstrate improved fusion rates, though concerns over heterotopic ossification and inflammatory responses necessitate continued refinement.
Maxillofacial Reconstruction
Defects resulting from tumor resection or trauma present unique challenges due to complex geometry. Customizable scaffolds loaded with BMPs and PDGF enhance bone fill and restore facial contours with fewer donor-site morbidities.
Emerging Technologies and Challenges
- Gene Therapy Approaches: Viral or non-viral vectors can induce local expression of growth factors but require safety validations to avoid off-target effects.
- 3D Bioprinting: Layered fabrication of cell-laden bioinks allows precise placement of MSCs and proteins, opening possibilities for patient-specific grafts.
- Smart Responsive Materials: Stimuli-responsive hydrogels or nanoparticles release growth factors in response to mechanical load or pH changes, mimicking physiological cues.
- Regulatory and Economic Hurdles: Manufacturing consistency, quality control, and high production costs slow widespread adoption.
Translational Outlook
Continued collaboration across biomaterials science, molecular biology, and clinical disciplines will be essential. Advances in imaging, computational modeling, and mechanobiology promise to refine scaffold design and growth factor dosing. By harnessing the synergistic power of stem cells, scaffolds, and signaling molecules, next-generation therapies aim to deliver robust, reliable bone regeneration for patients worldwide.