Bone healing represents a complex interplay of cellular activities, biochemical signals, and mechanical stimuli. This article addresses key pharmacological interventions designed to enhance natural repair mechanisms, highlighting advances that bridge basic science and clinical practice. Emphasis is placed on the molecular cascades governing tissue regeneration, the spectrum of therapeutic agents, and innovative delivery platforms.
Cellular and Molecular Basis of Bone Regeneration
Osteogenic and Angiogenic Pathways
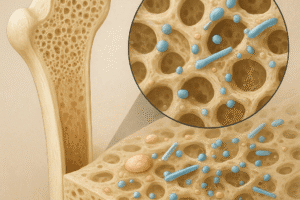
The process of bone repair relies heavily on osteogenesis and angiogenesis, two interdependent phenomena. After a fracture, hematoma formation initiates an inflammatory response, recruiting immune cells that secrete cytokines and growth factors. These mediators orchestrate the migration and proliferation of mesenchymal stem cells (MSCs) which differentiate into osteoblasts. Simultaneously, endothelial progenitor cells are mobilized to form new blood vessels, supplying oxygen and nutrients essential for callus formation.
Role of Osteoclasts and Remodeling
Bone remodeling requires a balance between bone formation and resorption. Osteoclasts, derived from hematopoietic lineage, resorb mineralized matrix, creating spaces for new bone. Factors such as receptor activator of nuclear factor kappa-B ligand (RANKL) and osteoprotegerin (OPG) regulate osteoclast differentiation. Dysregulation may lead to delayed healing or pathological conditions like nonunion.
Signaling Cascades and Therapeutic Targets
Key signaling pathways implicated in repair include BMP/Smad, Wnt/β-catenin, and Notch. For instance, bone morphogenetic proteins (BMPs) activate Smad transcription factors, driving osteoprogenitor commitment. Wnt proteins stabilize β-catenin, promoting expression of osteogenic genes. Modulation of these pathways offers targeted approaches to enhance bone regeneration.
Pharmacological Agents Modulating Bone Repair
Anabolic Therapies
Anabolic agents stimulate new bone formation through direct action on osteoblasts or upstream regulators. The most prominent examples include:
- BMP-2 and BMP-7: Recombinant growth factors approved for spinal fusion and long bone nonunions. They induce MSC differentiation and matrix synthesis.
- Parathyroid hormone(1-34): Intermittent administration enhances osteoblastic activity, increases trabecular bone volume, and accelerates fracture healing.
- Selective agonists of Wnt signaling: Investigational molecules that boost β-catenin levels, promoting an osteogenic phenotype in progenitor cells.
Anti-Resorptive Interventions
By limiting excessive bone breakdown, anti-resorptive drugs optimize the remodeling phase:
- Bisphosphonates: These agents bind hydroxyapatite and trigger osteoclast apoptosis. While effective in osteoporosis, timing is critical in fractures to avoid over-suppression of remodeling.
- Denosumab: A monoclonal antibody against RANKL, offering reversible inhibition of osteoclastogenesis, with potential use in bone defect repair.
- Calcitonin: Less potent but may reduce post-fracture pain and support early stabilization.
Adjunctive Small Molecules and Biologics
Emerging therapies include small molecules and biologics that fine-tune the microenvironment:
- Statins: Exhibit pleiotropic effects by upregulating BMP-2 expression and enhancing endothelial function.
- Angiogenic peptides: Promote neovascularization, crucial for nutrient delivery and waste removal.
- Sclerostin inhibitors: By targeting an osteocyte-secreted brake on bone formation, these antibodies boost osteoblastic synthesis.
Delivery Systems and Novel Therapeutic Strategies
Biomaterial Scaffolds and Carriers
Effective delivery of pharmacologics requires platforms that mimic the extracellular matrix and provide controlled release. Various biomaterials have been engineered to this end:
- Natural polymers: Collagen, chitosan, and alginate support cell adhesion and degrade into non-toxic byproducts.
- Synthetic polymers: Poly(lactic-co-glycolic acid) (PLGA) microspheres encapsulate growth factors, prolonging their activity.
- Hybrid composites: Incorporating bioactive ceramics like hydroxyapatite improves osteoconductivity and mechanical strength.
Gene Therapy and Cell-Based Approaches
Genetic modification of MSCs delivers sustained production of osteoinductive factors at the defect site:
- Viral vectors encoding cytokines or BMPs have shown promise in preclinical models.
- Non-viral delivery systems: Safer alternatives include liposomes and nanoparticles, though transfection efficiency remains a challenge.
- Engineered cell sheets: Cultured osteogenic cells layered on a carrier can be implanted directly into critical-size defects.
Smart and Responsive Hydrogels
Hydrogels that respond to environmental cues offer on-demand release of agents:
- pH-sensitive gels: Release payload when local acidity increases due to inflammation or bone turnover.
- Enzyme-responsive matrices: Degradation triggered by matrix metalloproteinases allows sequential delivery aligned with healing phases.
- Thermo-responsive polymers: Gelation at body temperature ensures minimally invasive injection and scaffold formation in situ.
Concluding Perspectives
Recent innovations in pharmacology and biomaterial science are converging to yield therapies that not only accelerate bone repair but also restore mechanical integrity. Future research aims to refine combination regimens, personalize treatments based on patient-specific biomarkers, and develop next-generation delivery vehicles capable of spatial and temporal control over healing signals.