Post-surgical bone healing represents a complex interplay of cellular events, mechanical forces, and therapeutic interventions aimed at restoring skeletal integrity. Effective optimization of this process is essential to reduce complications such as delayed union, nonunion, and functional impairment. Recent advances in **osteogenesis** research have illuminated strategies to enhance bone repair, including novel surgical techniques, targeted therapies, and personalized rehabilitation protocols.
Biological Foundations of Bone Regeneration
Bone healing unfolds in three overlapping phases: inflammation, repair, and remodeling. During the initial inflammatory phase, cytokines and immune cells clear debris and secrete mediators that recruit progenitor cells. The proliferative repair phase is marked by **angiogenesis**, where new blood vessels penetrate the fracture site, delivering oxygen and nutrients essential for callus formation. Osteoprogenitor cells then differentiate into osteoblasts, initiating **osteoconduction** and the deposition of woven bone. Finally, the remodeling phase replaces this immature bone with lamellar structure, restoring mechanical strength and microarchitecture.
Cellular and Molecular Drivers
- Mesenchymal stem cells (MSCs): multipotent cells that differentiate into osteoblasts and chondrocytes.
- Growth factors: bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF), and transforming growth factor-beta (TGF-β) orchestrate cellular proliferation and matrix synthesis.
- Extracellular matrix (ECM): scaffolding proteins like collagen type I provide a template for mineral deposition and bone maturation.
- Osteoclast-osteoblast coupling: tightly regulated by RANK/RANKL/OPG signaling to ensure balanced resorption and formation.
Surgical Interventions and Biomaterials
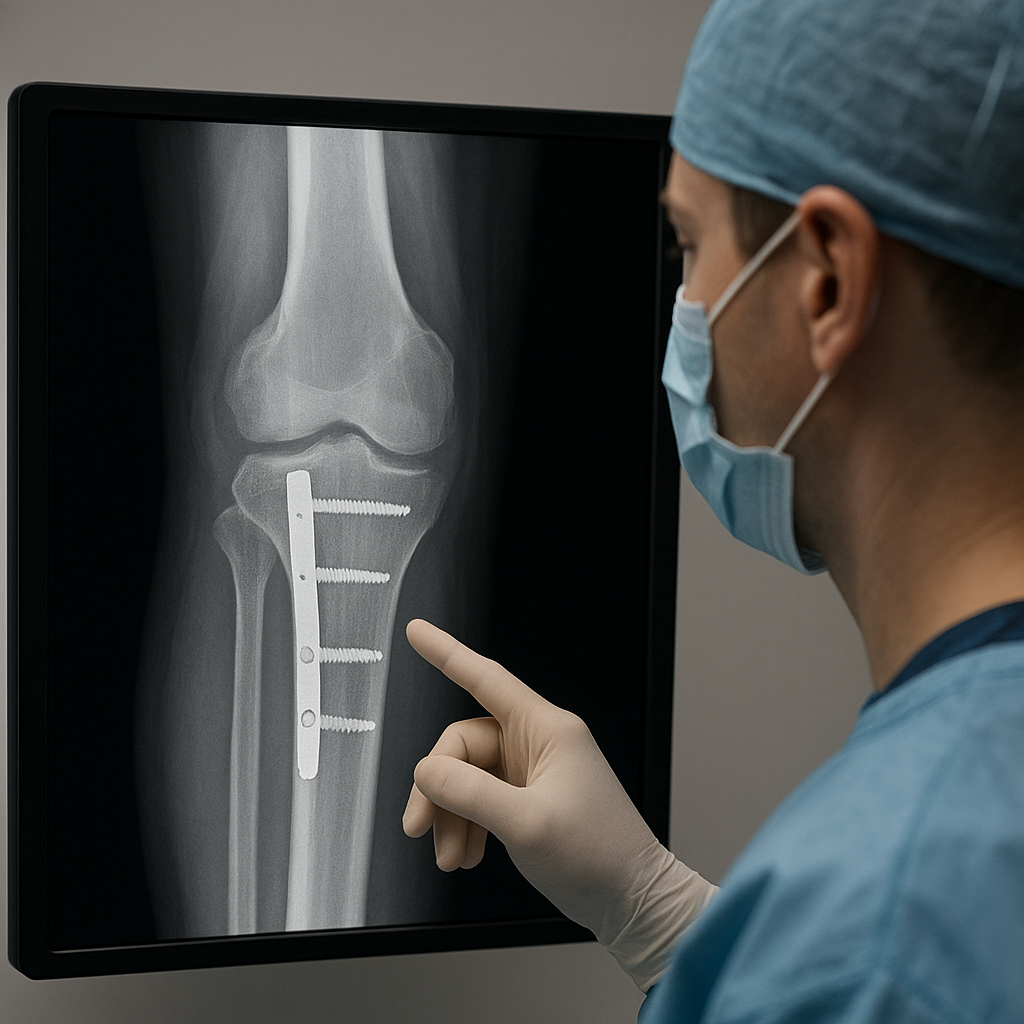
Optimizing post-surgical outcomes often relies on advanced fixation systems and graft substitutes. Rigid internal fixation maintains alignment, minimizing micromotion that can disrupt **osteointegration**. However, excessive rigidity may impede mechanical stimuli necessary for callus formation. Emerging devices utilize bioresorbable polymers or shape-memory alloys to provide dynamic support.
Autografts, Allografts, and Synthetic Scaffolds
- Autografts remain the “gold standard,” offering osteogenic cells and native growth factors but limited by donor-site morbidity.
- Allografts provide structural support but carry risks of immune reaction and disease transmission unless processed for **biocompatibility**.
- Synthetic **biomaterials**, such as hydroxyapatite, tricalcium phosphate, and bioactive glass, can be engineered with tailored porosity and degradation rates to mimic bone’s mechanical and biological properties.
- Composite scaffolds combining polymers and ceramics aim to optimize strength and osteoinductivity.
Pharmacological and Nutritional Strategies
Systemic and local pharmacotherapy can modulate bone metabolism. Nonsteroidal anti-inflammatory drugs (NSAIDs) alleviate pain but may impair early inflammatory signaling critical for **osteogenesis**. Bisphosphonates inhibit osteoclast-mediated resorption, potentially beneficial in osteoporotic patients but warranting caution to avoid oversuppression. Parathyroid hormone analogs (PTH 1-34) stimulate bone formation and have shown promise in accelerating callus maturation.
Micronutrients and Dietary Optimization
- Calcium and vitamin D: foundational for mineralization; supplementation is recommended when serum levels are deficient.
- Protein intake: essential amino acids support collagen synthesis and osteoblast function.
- Vitamin K2: enhances osteocalcin carboxylation, promoting bone matrix quality.
- Omega-3 fatty acids: anti-inflammatory properties may favor the initial healing environment.
- Magnesium and zinc: cofactors in enzymatic reactions for bone turnover.
Rehabilitation and Mechanical Stimulation
Controlled mechanical loading is a pivotal stimulus for callus consolidation. Early weight-bearing within tolerance can accelerate remodeling by activating signaling pathways responsive to strain. Physical therapy protocols integrate progressive resistance exercises to enhance muscle strength and joint stability, reducing the risk of post-operative stiffness and atrophy.
Techniques for Optimized Loading
- Low-intensity pulsed ultrasound (LIPUS): promotes **angiogenesis** and collagen alignment through mechanotransduction.
- Electrical bone growth stimulators: deliver capacitive or inductive currents that upregulate osteoblastic activity.
- Manual and aquatic therapy: utilize buoyancy and resistance to safely apply forces to healing bone.
- Customized orthoses: ensure proper alignment while permitting controlled micromotion.
Emerging Therapies and Future Directions
Innovative research is exploring tissue-engineered constructs and biologics to push the boundaries of bone repair. 3D bioprinting allows the fabrication of patient-specific scaffolds infused with **stem cells** and **growth factors**, potentially enabling in situ regeneration of complex defects. Gene therapy approaches aim to deliver BMP genes locally, sustaining osteoinductive signals over extended periods. Nanotechnology-based carriers provide targeted release of therapeutic agents, optimizing local concentrations and minimizing systemic side effects.
Personalized Medicine and Digital Health
- Biomarker profiling: genetic and proteomic data to predict individual healing potential and tailor interventions.
- Wearable sensors: continuous monitoring of load distribution and limb alignment to guide rehabilitation progress.
- Machine learning algorithms: integrate patient data to forecast healing trajectories and optimize treatment plans.
- Telemedicine platforms: enable remote supervision of physiotherapy, improving adherence and outcomes.