The process of bone fracture healing is a remarkable example of the body’s innate capacity for regeneration and repair. When a bone breaks, a well-orchestrated sequence of cellular and molecular events begins, restoring structural integrity and returning function. Understanding each stage of this process provides valuable insights for clinicians, researchers, and patients alike.
Inflammatory Phase
Immediately after a fracture, the body initiates the inflammatory response. Blood vessels at the injury site rupture, forming a hematoma. This clot serves as a provisional matrix and releases key signaling molecules.
Cytokine and Growth Factor Release
- Cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) attract immune cells.
- Platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-β) stimulate mesenchymal stem cell recruitment.
- Macrophages and neutrophils clear debris and secrete additional signaling factors.
Vascular Response
Within hours, new capillaries begin to sprout, a process known as angiogenesis. This vascular ingrowth is critical for delivering oxygen, nutrients, and cells to the fracture site.
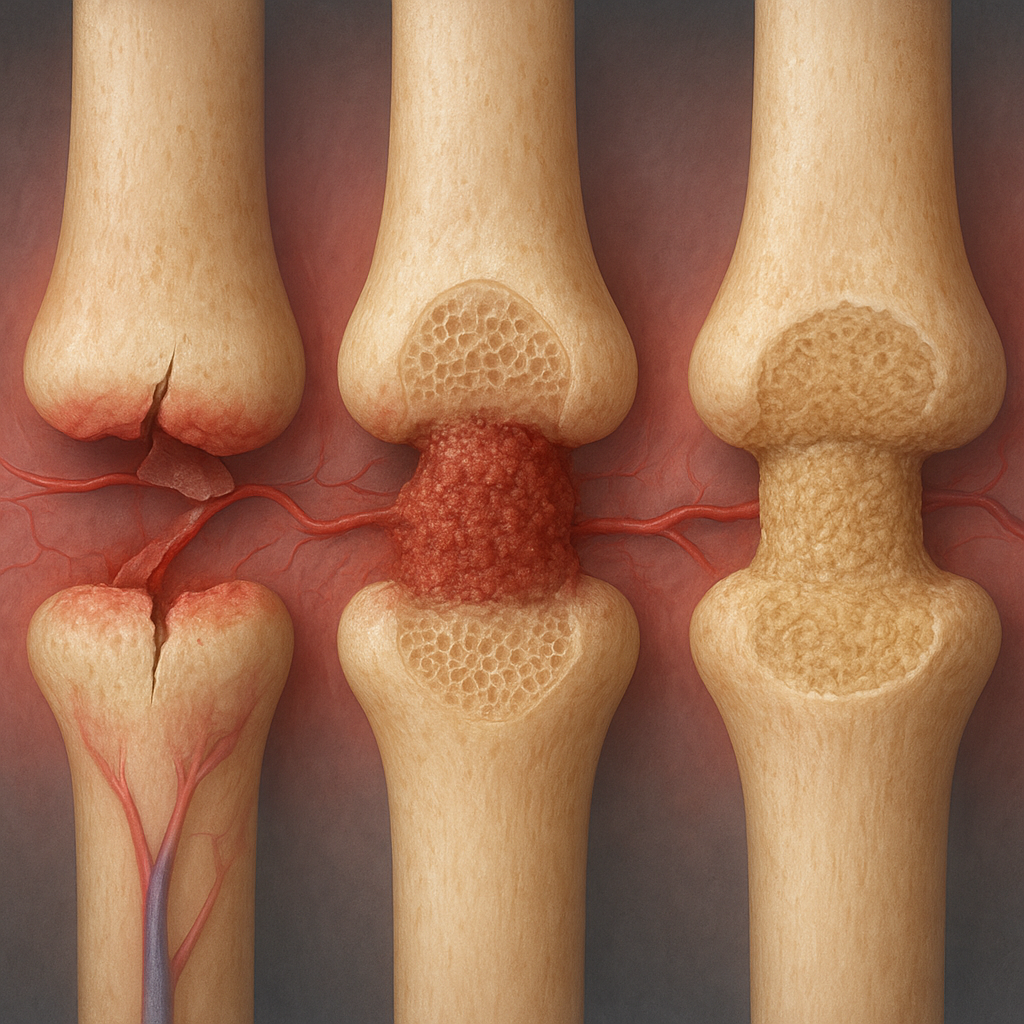
Repair Phase
The repair phase can be divided into two overlapping events: soft callus formation and hard callus formation. Both are essential for bridging the fracture gap and regaining mechanical strength.
Soft Callus Formation
- Mesenchymal stem cells differentiate into chondroblasts, producing a cartilaginous matrix.
- This fibrocartilaginous callus stabilizes the fracture while it is still flexible.
- Ongoing angiogenesis increases local blood flow, enabling further cell recruitment.
Hard Callus Formation
As the process continues, chondroblasts die off and are replaced by osteoblasts. These bone-forming cells deposit woven bone, converting the soft callus into a hard bony callus.
- Woven bone is disorganized but rapidly produced, providing initial mechanical stability.
- Mineralization occurs as hydroxyapatite crystals deposit within the collagen matrix.
- By the end of this phase, the fracture can bear weight, though strength remains below normal.
Remodeling Phase
The final stage of healing, remodeling, restores bone to its original shape and strength. This lengthy process may continue for months to years.
Role of Osteoclasts and Osteoblasts
- Osteoclasts resorb excess woven bone and reshape the callus.
- Osteoblasts lay down new lamellar bone in a highly organized pattern.
- Mechanical stresses guide remodeling through Wolff’s law, ensuring that bone architecture matches functional demands.
Restoration of the Medullary Cavity
During remodeling, osteoclasts carve out the central marrow space, recreating the original medullary cavity. Blood supply and marrow elements fully return, completing the healing cycle.
Factors Influencing Fracture Healing
Multiple intrinsic and extrinsic factors can accelerate or delay healing. Awareness of these elements is essential for optimizing clinical care.
Intrinsic Factors
- Patient age and hormonal status: younger individuals and those with healthy endocrine function heal faster.
- Nutritional status: adequate levels of calcium, vitamin D, and proteins support bone matrix formation.
- Comorbidities: diabetes, osteoporosis, and vascular diseases can impair blood flow and cellular activity.
Extrinsic Factors
- Stability and fixation: rigid internal fixation vs. functional bracing influences callus size and remodeling pace.
- Mechanical loading: controlled weight-bearing promotes proper alignment and stimulates remodeling.
- Medications: nonsteroidal anti-inflammatory drugs (NSAIDs) and certain antibiotics may interfere with inflammation and cell proliferation.
Advanced Therapies and Future Directions
Recent advances in biotechnology are expanding the therapeutic toolbox for challenging fractures. Investigational strategies aim to enhance or mimic the natural healing cascade.
Cell-Based Therapies
- Autologous mesenchymal stem cell transplantation offers targeted delivery of regenerative cells.
- Genetically modified cells producing growth factors such as bone morphogenetic proteins (BMPs) show promise in preclinical studies.
Biomaterials and Scaffolds
Engineered scaffolds composed of biodegradable polymers and ceramics provide structural support while releasing bioactive molecules. These constructs promote cellular adhesion, proliferation, and differentiation at the fracture site.
Biophysical Stimulation
- Low-intensity pulsed ultrasound (LIPUS) enhances callus formation by stimulating cellular activity.
- Electrical and electromagnetic fields can modulate osteogenic pathways and improve mineralization.
Clinical Assessment and Monitoring
Accurate evaluation of fracture healing guides treatment decisions and rehabilitation planning.
Imaging Techniques
- X-rays remain the gold standard for assessing callus formation and alignment.
- Computed tomography (CT) provides detailed visualization of bone bridging and cortical continuity.
- Magnetic resonance imaging (MRI) can evaluate soft tissue involvement and early vascular changes.
Biomarkers of Healing
Emerging research investigates serum and urinary biomarkers, such as alkaline phosphatase and N-terminal propeptide of type I collagen (P1NP), to quantify bone formation rates and predict healing outcomes.
Rehabilitation and Functional Recovery
Effective rehabilitation protocols are tailored to the healing stage, balancing protection with progressive loading.
Early Phase Strategies
- Immobilization or limited motion to protect the fracture site.
- Pain management and edema control using cryotherapy and elevation.
- Isometric exercises to preserve muscle tone and prevent joint stiffness.
Late Phase Strategies
- Controlled weight-bearing and resistance exercises to stimulate remodeling.
- Proprioceptive training to restore neuromuscular coordination.
- Functional milestones: gait normalization, return to sport-specific activities, and occupational demands.