Bone grafting has been a cornerstone of reconstructive surgery, addressing defects from trauma, disease, and congenital malformations. By harnessing the body’s capacity for skeletal repair, clinicians employ a variety of graft materials and techniques to restore function and structure. This article reviews key classifications, surgical considerations, clinical applications, and future innovations in the field of bone transplantation.
Classification of Bone Grafts and Their Properties
Bone graft materials are broadly categorized according to their **origin** and **functional** properties. A thorough understanding of these features allows surgeons to select the most suitable graft for each clinical scenario.
Autografts
- Definition: Tissue harvested from the patient, often from the iliac crest, tibia, or fibula.
- Advantages: Superior osteogenic potential, no risk of immunogenic rejection, and high biocompatibility.
- Limitations: Donor site morbidity, limited volume, and prolonged surgical time.
Allografts
- Definition: Bone obtained from a genetically different donor of the same species, processed in tissue banks.
- Advantages: Larger volumes available and no donor site complications.
- Limitations: Reduced osteoinductive capacity after processing, risk of disease transmission, and possible immunogenic response.
Xenografts and Synthetic Grafts
- Xenografts: Bone harvested from other species (usually bovine), extensively treated to remove antigens.
- Synthetic Grafts: Engineered materials such as calcium phosphates, bioactive glass, and polymer composites.
- Key Features: High structural versatility, ease of sterilization, and customizable degradation rates. Many synthetic scaffolds are designed to mimic the porous architecture of native bone, facilitating vascular ingrowth and osteoconduction.
Graft materials are further assessed by three core functions: osteogenesis (new bone formation by living cells), osteoconduction (scaffold for vascular and cellular invasion), and osteoinduction (stimulation of progenitor cell differentiation). The ideal graft integrates all three, although few materials possess complete tri-functionality without combining multiple sources or additives.
Techniques and Surgical Considerations
Successful grafting demands meticulous surgical planning, precise technique, and awareness of biological principles. Surgeons must evaluate defect size, mechanical stability, vascular supply, and patient-specific factors.
Preoperative Planning
- Imaging modalities such as CT and MRI reveal defect geometry and host bone quality.
- 3D printing and virtual surgical planning offer patient-specific guides to optimize graft shape and alignment.
- Preoperative assessment of comorbidities (e.g., diabetes, smoking) that impair healing.
Graft Handling and Preparation
- Autograft harvesting techniques minimize donor site trauma and preserve cellular viability.
- Allograft processing ensures sterility while retaining critical growth factors; gamma irradiation or chemical treatments must balance decontamination with preservation of biological activity.
- Synthetic scaffolds may be combined with growth factors such as BMP-2 or platelet-rich plasma to enhance osteoinduction.
Fixation and Stabilization
- Rigid internal fixation (plates, screws, intramedullary nails) is essential for load-sharing and prevention of micromotion at the graft-host interface.
- External fixators can be employed for complex deformities or infected sites, facilitating gradual distraction or compression osteogenesis.
- Biodegradable fixation devices (e.g., magnesium alloys, PLLA) are under investigation to eliminate the need for secondary removal surgery.
Adjunctive measures such as local antibiotic delivery, hyperbaric oxygen therapy, and negative pressure wound therapy can further optimize the biological environment for regeneration.
Clinical Applications in Orthopedics and Dentistry
Bone grafting plays a pivotal role across multiple specialties, from large skeletal reconstructions to fine dental procedures.
Orthopedic Trauma and Reconstruction
- Segmental defects after high-energy fractures or tumor resection often exceed the regenerative capacity of native bone.
- Vascularized fibular grafts combine autogenous bone with microvascular anastomosis, providing living tissue capable of hypertrophy under load.
- Masquelet technique employs a two-stage induced membrane to encapsulate a cement spacer, followed by autologous bone grafting within a biologically active cavity.
Spinal Fusion and Degenerative Disorders
- Interbody fusion cages filled with bone graft or bone marrow aspirate restore disc height and biomechanical stability.
- Bone morphogenetic proteins (BMPs) have gained approval for select spinal applications, though off-label use and potential complications warrant cautious consideration.
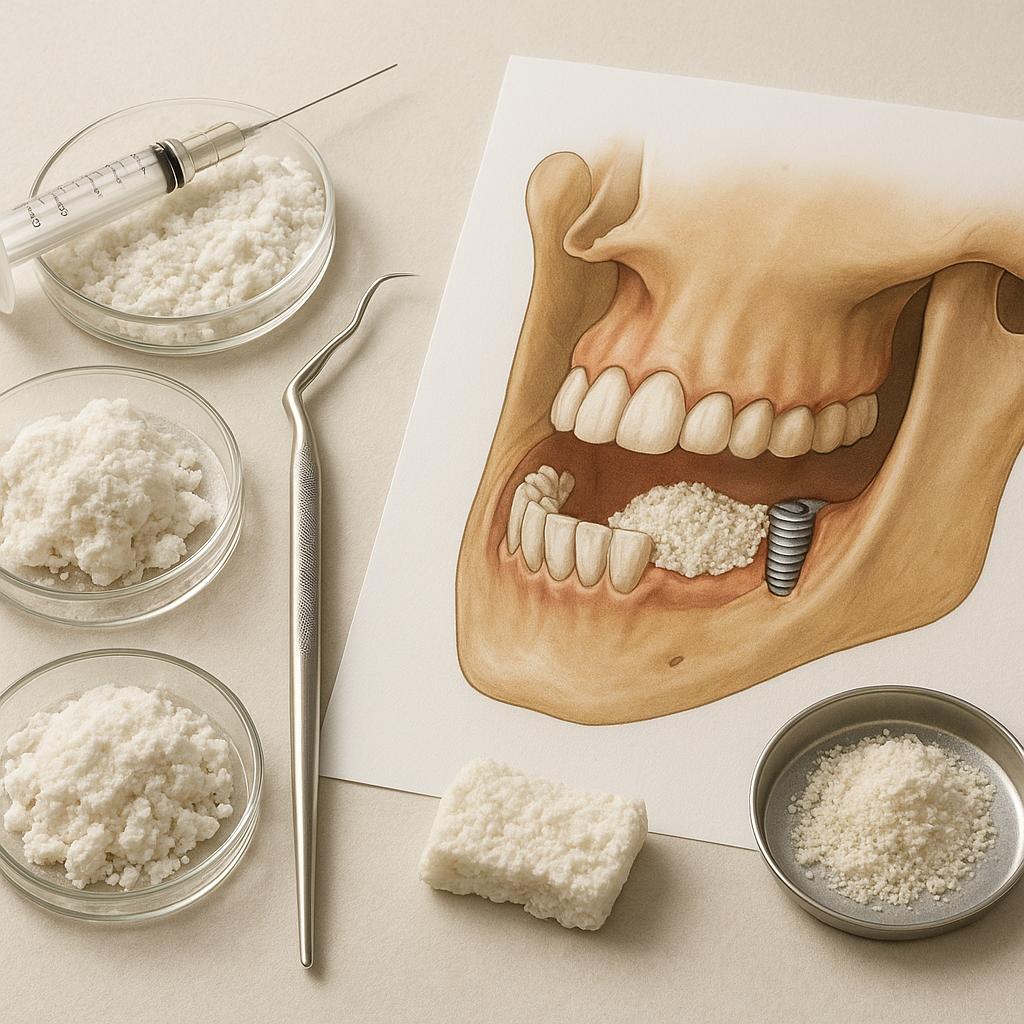
Maxillofacial and Dental Implantology
- Alveolar ridge augmentation and sinus lifts prepare the jaw for endosseous implants, preventing implant failure due to insufficient bone volume.
- Guided bone regeneration employs barrier membranes to exclude soft tissue ingrowth, allowing selective osteogenic colonization.
- Distraction osteogenesis in the mandible and maxilla gradually lengthens bone, reducing the need for large-volume grafts.
Outcomes in these diverse fields underscore the importance of matching graft type to mechanical demands, anatomical constraints, and the patient’s healing capacity.
Emerging Trends and Future Perspectives
The landscape of bone grafting is rapidly evolving, driven by advances in materials science, cellular therapies, and biofabrication.
Stem Cell–Based Strategies
- Mesenchymal stem cells (MSCs) from bone marrow, adipose tissue, or umbilical cord are seeded onto scaffolds to augment osteogenesis.
- Exosomes and extracellular vesicles derived from MSCs may offer a cell-free alternative to stimulate bone repair without the complexity of live cell transplantation.
Bioprinting and Customized Scaffolds
- Three-dimensional bioprinting techniques allow deposition of bioinks composed of hydrogel, ceramic particles, and viable cells in precise architectures.
- Patient-specific implants, designed from imaging data, ensure perfect anatomic fit and controlled porosity to guide vascular ingrowth.
Smart Biomaterials
- Stimuli-responsive materials release growth factors or antibiotics in response to pH changes or mechanical loading.
- Nanostructured coatings and surface modifications can improve cell adhesion, proliferation, and antimicrobial defense.
Integration of digital technologies, from intraoperative navigation to artificial intelligence–driven planning, promises enhanced accuracy and personalized therapy. As regulatory frameworks adapt to complex biologics and combination products, rigorous clinical trials will be essential to validate safety and efficacy.
The interplay of **biology**, **engineering**, and **clinical expertise** continues to drive innovation in bone grafting. By leveraging autologous cells, synthetic scaffolds, and cutting-edge manufacturing, the field moves closer to the goal of fully functional skeletal regeneration.