Bone health is a complex interplay between cellular activity, nutritional factors, and endocrine signals. This article explores the vital connection between hormones and skeletal strength, shedding light on the mechanisms through which hormonal fluctuations influence bone formation and resorption. By understanding the roles of key endocrine regulators, we can appreciate how imbalances may result in compromised bone quality and increased fracture risk. This knowledge informs targeted medical interventions and supports preventive lifestyle strategies aimed at preserving skeletal integrity throughout life.
Hormonal Regulation of Bone Metabolism
The adult skeleton undergoes continuous remodeling to maintain structural integrity. Specialized cells known as osteoblasts build new bone, while osteoclasts break down old tissue. These opposing activities remain in dynamic homeostasis under the influence of systemic hormones. When the balance shifts toward resorption, bone mass declines; when formation predominates, bone density increases. Dysregulation of this equilibrium contributes to conditions such as osteoporosis and osteopenia, highlighting the necessity of precise endocrine control.
The bone remodeling cycle consists of activation, resorption, reversal, and formation phases. It begins when osteoclast precursors are recruited and differentiate under hormonal cues. Following matrix degradation, osteoblast precursors are signaled to synthesize organic bone matrix, which then mineralizes. Hormones modulate each stage by altering gene expression, cell proliferation, and apoptosis rates. Understanding these molecular pathways underpins the development of therapies aimed at restoring skeletal balance in patients with metabolic bone disease.
Cellular receptors sense circulating levels of key hormones and convey signals through intracellular pathways. For instance, cyclic AMP and Wnt signaling cascades translate hormonal binding events into transcriptional changes, thereby controlling cell fate. The interplay between mechanical loading, local growth factors, and endocrine inputs further refines bone adaptation, ensuring that the skeleton can respond to both physiological demands and hormonal variations over the lifespan.
Estrogens and Androgens: Guardians of Bone Integrity
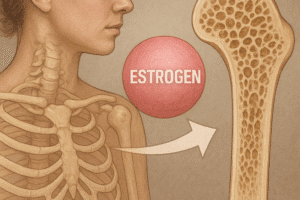
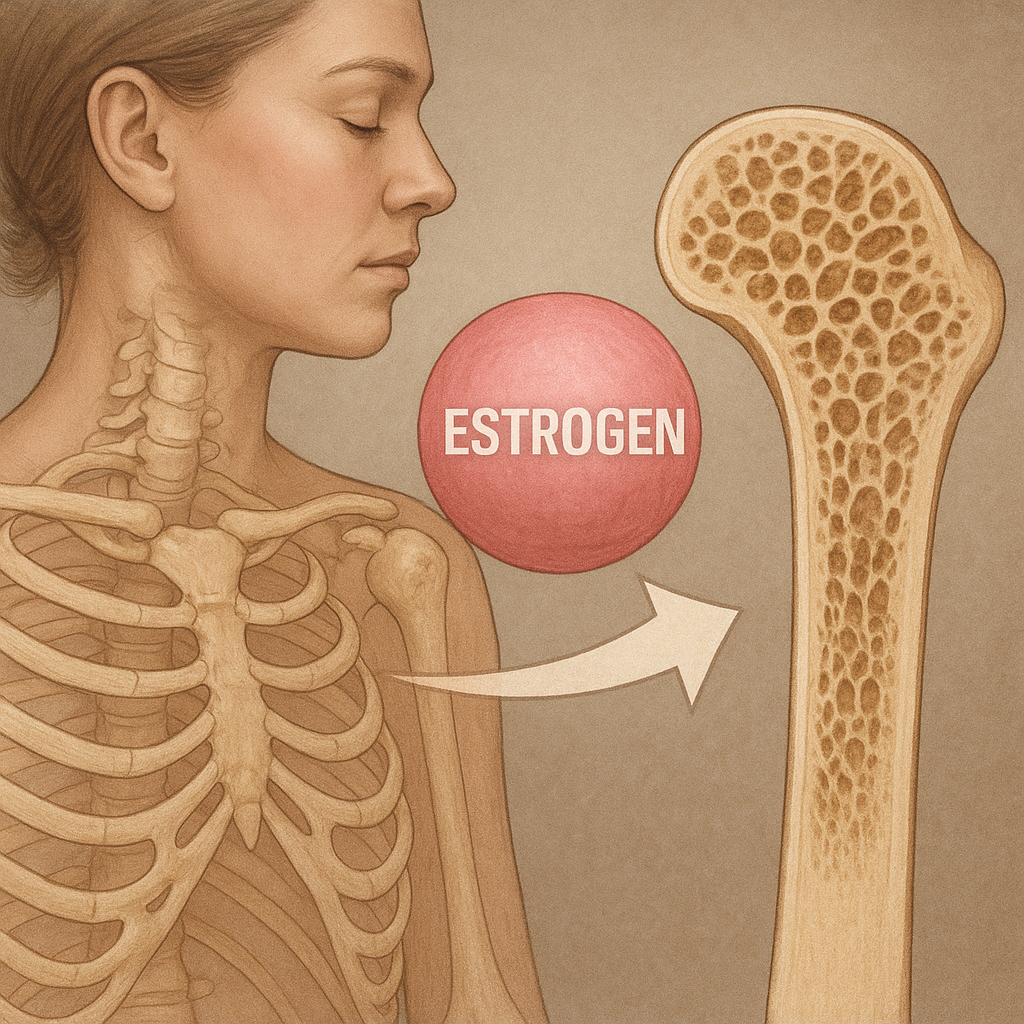
Sex steroids play a pivotal role in maintaining bone mineral density. Estrogen deficiency after menopause accelerates bone resorption by increasing osteoclast lifespan and activity. In men, decline in androgen production gradually reduces bone formation, though at a slower rate compared to postmenopausal women. Both estrogens and androgens suppress inflammatory cytokines that promote resorption, while enhancing osteoblast differentiation and survival. Their combined effects are essential for peak bone mass attainment during adolescence and for preserving bone strength in adulthood.
At the molecular level, estrogen receptors (ERα and ERβ) in osteoblasts and osteoclasts regulate gene networks that control apoptosis and matrix synthesis. Androgen receptor signaling in osteoblasts stimulates collagen production and mineral deposition. Therapeutic use of hormone replacement can mitigate bone loss, but long-term safety considerations—such as cardiovascular risks or hormone-sensitive cancers—require careful patient selection and monitoring. Novel selective receptor modulators aim to harness the skeletal benefits of sex steroids while minimizing unwanted effects on other tissues.
Research into gender-specific bone health emphasizes that men and women may respond differently to hormonal therapies. While women often benefit from low-dose estrogen regimens initiated near menopause, men with hypogonadism may require individualized androgen replacement plans. Ongoing clinical trials are evaluating the efficacy of combined estrogen–androgen therapies, as well as the impact of timing and dosage on long-term fracture risk reduction.
The Role of Parathyroid and Growth Hormone
Parathyroid hormone (PTH) is secreted in response to low serum calcium levels. Chronic elevation of PTH induces bone resorption to restore calcium homeostasis, whereas intermittent, low-dose administration exerts anabolic effects by stimulating osteoblast proliferation. This paradoxical response led to the development of PTH analogues as potent anabolic therapies for severe osteoporosis. Daily injections of recombinant PTH enhance bone formation more than resorption, resulting in net bone gain and reduced fracture risk.
Growth hormone (GH) and its downstream mediator, insulin-like growth factor 1 (IGF-1), also influence skeletal development and maintenance. GH promotes chondrocyte proliferation in the growth plate, driving longitudinal bone growth in children and adolescents. In adults, GH–IGF-1 axis activity supports osteoblast differentiation and collagen synthesis. Decline in GH secretion with aging contributes to reduced bone formation, highlighting potential benefits of GH replacement in select individuals, although concerns over adverse metabolic effects have limited widespread use.
Balancing PTH and GH signaling is crucial: excessive endogenous PTH leads to cortical bone loss, whereas GH excess, as seen in acromegaly, results in disorganized bone architecture. Conversely, PTH deficiency impairs calcium regulation, and GH deficiency compromises bone turnover. Future treatments may combine targeted modulation of these axes to optimize bone quality without provoking systemic imbalances.
Clinical Implications and Hormone-Based Therapies
Advances in endocrinology have translated into a diverse arsenal of hormone-based treatments for bone disorders. Beyond traditional bisphosphonates, which inhibit osteoclast-mediated resorption, clinicians now can prescribe PTH analogues, selective estrogen receptor modulators (SERMs), and monoclonal antibodies against key signaling proteins such as RANKL. Each agent targets a distinct hormonal or paracrine pathway, allowing personalized therapy based on a patient’s endocrine profile and fracture history.
Monitoring serum levels of estradiol, testosterone, PTH, and vitamin D metabolites helps tailor interventions. For example, in postmenopausal osteoporosis, combining calcium and vitamin D supplementation with SERMs may preserve bone density without the risks associated with systemic estrogen. In male hypogonadism, low-dose testosterone therapy can improve bone mass and muscle strength, although potential cardiovascular and prostate health implications must be weighed.
Emerging strategies include peptide mimetics that activate anabolic signaling without catabolic side effects, and gene therapies aimed at enhancing local production of growth factors. Precision medicine approaches integrate genetic screening—identifying polymorphisms in hormone receptor genes—with imaging techniques to predict individual response, ushering in an era of more effective and safer bone-strengthening protocols.
Lifestyle Factors and Hormonal Balance
Optimal bone health depends not only on pharmaceutical interventions but also on lifestyle factors that influence endocrine function. Adequate intake of protein, calcium, and vitamin D supports hormone synthesis and skeletal mineralization. Weight-bearing exercise stimulates local release of growth factors and promotes sensitivity to circulating hormones. Conversely, chronic stress and poor sleep can disrupt cortisol rhythms, leading to increased bone resorption and decreased formation.
Alcohol overconsumption and smoking impair sex steroid production and reduce the bioavailability of vitamin D, compounding hormonal deficits. Maintaining a balanced body weight is crucial: underweight individuals may experience amenorrhea and low estrogen levels, while obesity can alter leptin signaling, indirectly affecting bone remodeling. Nutrition and exercise programs should be integrated into treatment plans to maximize the benefits of endocrine therapies.
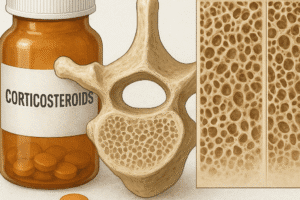
Early identification of hormonal risk factors through routine screening in at-risk populations—such as postmenopausal women, men with hypogonadism, and patients on long-term glucocorticoids—enables timely lifestyle modifications and medical management. By combining healthy habits with targeted hormonal interventions, clinicians can uphold skeletal strength and reduce the burden of fractures across the lifespan.